SciMeditor
Biomedical writing and editing
Helping physicians and scientists publish their work for over 25 years
Medical writing services
Physicians have a formidable workload and sometimes would like help writing up their medical research.
I did my PhD and postdocs in the field of immunology and infectious and autoimmune diseases, during which time I wrote 11 of my own articles. Subsequently, I worked with non-native English-speaking physicians and scientists, helping them to publish >3500 articles and meet other career goals. More recently, I have helped with writing papers, which has in some instances doubled author output in high-ranking journals. Thus, I have considerable experience in helping busy physicians publish well.
This medical-writing work can start from various points in the manuscript preparation process. For example, I can write the paper on the basis of the data analyses, the protocol/summary of the study objectives, and a list of the most relevant references.
Alternatively, the physician writes a rudimentary paper in the IMRAD format (Introduction-Methods-Results-and-Discussion) and I flesh it out into a well-argued and solid paper that is firmly grounded in the background literature and meets the relevant biomedical reporting guideline and journal requirements. A cover letter will also be provided and all data display items will be formatted according to journal guidelines. Figures can also be generated as needed.
The physician and his/her team is involved in every single step of the work I do. I may provide ideas for further analyses, directions, arguments, and/or appropriate journals. All decisions will be made by the client.
In addition, I provide post-submission services, including writing rebuttal letters and revising the paper to address reviewer comments. I will continue working with you until your paper is published!
Cost depends on the requirements of the work. If you are interested in this service and would like a free quote, please contact me on SciMeditor@gmail.com.
Please note that ghostwriting - meaning writing a scientific paper and not being appropriately credited - is unethical and strongly discouraged by regulatory bodies such as the International Committee of Medical Journal Editors (ICMJE). If my contributions meet the current norms of scientific authorship, it will be necessary to add my name to the author list.
Editing packages
Three options to suit your editing needs
Basic Editing package
Simple spelling and grammar check for accepted papers needing a final grammar check
Learn MoreComprehensive PLUS Ongoing Support Until Submission package
Comprehensive editing with word count reduction and UNLIMITED revisions until submission.
Learn MoreAnalytical Review Service
An Analytical Review reveals where your paper could be strengthened, thereby improving its chances of passing peer review
When is it useful to order an Analytical Review of your paper?
A common complaint of authors is that they have worked so long on their manuscript that they lose their overall perspective on it. They wonder, is the message of my paper coming across consistently, accurately, and clearly? Have I structured my paper properly? Have I included all the relevant details? Or have I included too much detail? Will the reviewers understand immediately how my study contributes significantly to the field? Have I made any glaring mistakes that will cause the journal editors and peer reviewers to view my manuscript unfavorably?
In such cases, it can be helpful to ask me to write an Analytical Review of your paper. This involves me first comprehensively editing your paper and then critically analyzing it according to a detailed list of criteria to identify areas that require improvement during revision, including:
- is the key message clearly and consistently transmitted throughout the paper?
- how valid and original is the scientific question that is being asked?
- are the study design and methodology appropriate for the study question?
- are the data convincing?
- are the conclusions warranted?
- do the concepts in the study report flow naturally and logically?
- does the study report contain all the elements recommended by the relevant study guideline (e.g. CONSORT, STROBE, ARRIVE etc.)
- are the data descriptions consistent and coherent?
- are there any other major or minor issues that might cause a journal editor or peer reviewer to view your paper unfavorably?
- is the selected journal appropriate for your paper?
The outcome of an Analytical Review is a report that starts with a summary of the study objective, design, key findings, and main conclusions. The report then notes all of the weaknesses and inconsistencies in the manuscript and provides recommendations on how to improve it. The report is sent to you along with the edited documents.
Price of an Analytical Review:
US$240
Are you interested in an Analytical Review of your paper after it is edited? If so, please go to the Editing Service Order Form and tick the "Yes" box at the question "Would you like to order an Analytical Review?"
How to order editing services
It's super easy!
The ordering process is described in full in the Detailed ordering information page but here's a quick overview.

Choose whether the
best fits your needs
Complete the order form, including whether you
wish an Analytical Review of your work
Click SEND at the bottom of the order form

Within 24 hours, I will confirm your choices by email and
ask for the go-ahead to start work

manuscript
I will send you an email with the completed work
and instructions about how to pay

You can pay your invoice
by credit card or bank transfer
Click here to learn more about the ordering process
OR
Click the button below to go to the Editing Service Order Form
Types of documents edited
- biomedical research papers on humans (randomized clinical trials, observational studies, case series, case reports)
- human and animal laboratory research papers (fundamental and applied science)
- biotechnology papers
- environmental, occupational health, and public health research papers
- zoological research papers
- reviews, systematic reviews, and meta-analyses
- abstracts
- letters to the editor
- cover letters
- medical and scientific books, book chapters, and university text books
- grant applications
- responses to reviewers and rebuttal letters
- conference materials (programs, slide decks and presentations, abstracts, posters, conference reports)
- institution report abstracts
- research proposals, laboratory reports, project summaries, promotional material, CVs, job applications, correspondence, and website text.
Choose the package that is most suitable for you:
Or go directly to the Editing Service Order Form:
Why choose SciMeditor?
Experienced medical writing and editing that greatly increases the chance of your valuable research being published in impactful journals
Academic writing is difficult: extensive experience and strong language skills are needed to ensure that the key messages of your research are expressed clearly, accurately, and convincingly. Along with funding, English language and writing proficiency are the most important factors that shape your publication chances in high-ranking English language journals.1 I am an excellent choice as your medical writer and editor for many reasons, in particular:
(1) Extensive skills and experience
I (the founder of SciMeditor) am a PhD-qualified medical writer who has a strong background in biomedical research, outstanding English language skills, and a strong commitment to excellence. I have clients based all over the world and extensive experience: over the last 20 years, I have worked on:
* >3500 biomedical papers
* >600 rebuttal letters
* >100 grant applications.
(2) A proven track record of helping researchers publish quickly in excellent journals
In the last decade:
* More than doubled the publication rate of individual authors in high-ranking journals
* 41% of all papers I edited were published in the top 10% of journals in the field
* Mean (median) Journal IF of the publication journals = 3.918 (2.878)
* Mean (median) SNIP of the publication journals = 1.301 (1.131)
* Journals include The Lancet, Nature Medicine, Nature Communications, Annals of the Rheumatic Diseases, Gut, Cell Metabolism, PNAS, JEM, JACC...
* Average duration from first edit to publication = 6 months
* 91% of papers published in 12 months
Thus, I can help you to produce polished, concise, highly readable, persuasive, and very clear manuscripts that stand an excellent chance of rapid publication in the best journal for your work. I guarantee you that your edited documents will not be rejected because of language or structural problems.
(3) Multiple services for differing needs
My extensive experience with my clients has led me to develop a variety of services that will meet your every need as you move towards successful and career-affirming publication. Thus, you can choose between:
* Medical writing of original research papers
* Three editing packages that can be used for any type of scientific document. The packages offer:
- Two turnover rates: Regular (7 days) and Express (72 hours)
- Two levels of editing: Basic and Comprehensive
- Two levels of FREE revision support: Comprehensive (one FREE re-edit) and Comprehensive PLUS (unlimited FREE re-edits until publication).
Comprehensive PLUS is the most generous editing package on the web.
* My Analytical Review service can also show you how to further strengthen your manuscript before it goes for peer review.
(4) Excellent and personal service that has satisfied many clients over the years
"she is an exceptional writer"
"superb editing skills"
"never experienced such high quality work"
"outstanding editor"
"brilliant"
"gifted at seeing the big picture"
"careful and accurate"
"advanced and serious"
"best I have ever seen"
"important comments and questions"
"exhaustive and excellent"
"always satisfied"
"perfect"
"accepted without changes needed"
About me
I truly love what I do: I really enjoy reading your research, working with you, and helping you successfully produce impactful manuscripts
 I established SciMeditor to help physicians and researchers to achieve important career-affirming aims such as publishing their work in high-impact journals and obtaining research funds.
I established SciMeditor to help physicians and researchers to achieve important career-affirming aims such as publishing their work in high-impact journals and obtaining research funds.
I am passionate about advancing the cause of science and medicine and feel that equitable global partnership that is unhindered by language difficulties best achieves this ideal.
I have 11 years of experience in well-reputed scientific laboratories and more than 20 years of experience in writing and editing scientific and medical research manuscripts and other documents (>3500 manuscripts). I am committed to excellent science and will help you to produce elegant professional work that is clear, concise, accurate, and highly readable.
Your investment in my services will greatly improve your chances of rapid publication in high-quality journals and of receiving funding. My ultimate goal is to help you to pursue a long, impactful, and successful career.
Academic and professional background

CURRENT PROFESSION
SciMeditor Founder and Medical Writer and Editor, 2002–present.
(i) Edited >3500 biomedical research papers and book chapters, >600 reply-to-reviewer letters, and >100 grant applications.
(ii) Wrote >350 Analytical Reviews of research articles to guide revision before publication.
(iii) Wrote multiple papers with clients (see Research Publications below).
- Biology (immunology, microbiology, molecular biology, biotechnology, zoology)
- Medicine (anesthetics, cardiology, dermatology, diagnostic medicine, ENT, emerging diseases, emergency medicine, endocrinology, gastroenterology, hematology, immunology, intensive care medicine, microbiology, neurology, nuclear medicine, occupational health, occupational therapy, oncology, ophthalmology, orthopedics, plastic and reconstructive surgery, public health, nephrology, rheumatology, sports medicine, surgery, urology, women's health, veterinary medicine)
- Dentistry (orthodontics, prosthodontics, oral & maxillofacial surgery)
- Epidemiology
UNIVERSITY EDUCATION
PhD in Tropical Medicine and Immunology, 1990–1993. Thesis: CD4+ T-cell responses to the circumsporozoite proteins of Plasmodium falciparum and P. vivax by adults living in endemic and non-endemic regions of Thailand. Queensland Institute of Medical Research and University of Queensland, Brisbane, Australia. Field research was performed in the Research Institute of Health Sciences (RIHES), Chiang Mai, Thailand.
BSc (Honors) in Tropical Medicine and Immunology, 1989. Thesis: CD4+ T-cell responses to the circumsporozoite protein of Plasmodium falciparum by malaria-exposed Caucasians. Queensland Institute of Medical Research and University of Queensland, Brisbane, Australia.
Bachelor of Science, 1985–1988. Majors in Biochemistry and Zoology. University of Queensland, Brisbane, Australia.
PREVIOUS PROFESSIONAL EXPERIENCE
Postdoctoral fellow, 1998–2002. Netherlands Cancer Institute, Amsterdam, The Netherlands. Effect of expressing myelin basic protein in antigen-presenting cells on T-cell tolerance in experimental autoimmune encephalomyelitis.
Postdoctoral fellow, 1996–1998. Max-Planck Institute for Infection Biology, Berlin, Germany.
(1) Role of commensal Neisseria species in the development and progression of T-cell autoimmunity in rheumatoid arthritis and other arthritides.
(2) Development of vaccines against Helicobacter pylori.
Postdoctoral fellow, 1994-1995. Queensland Institute of Medical Research, Brisbane, Australia. Effect of natural polymorphisms in immunodominant T-cell epitopes in the circumsporozoite protein of Plasmodium falciparum on peripheral blood T-cell responses.
ADDITIONAL EDUCATION
Workshops at European Medical Writers Association (EMWA) conferences 2011–2015, including:
| Guide to key clinical documents | Documents from protocol to study report |
| Writing the clinical study protocol | Writing clinical study reports using ICH E3 |
| Subject narratives for medical writers | Good Clinical Practice (GCP) training |
| Clinical study appendices | Drug safety for medical writers |
| From clinical study report to manuscript | Medical devices |
| Development Safety Update Reports | Grant writing |
| Critical appraisal of medical literature | Analysis of variance and regression analysis |
| Advanced epidemiology |
Obtained EMWA Professional Development Programme (EPDP) Certificate in Drug Development Writing in 2015.
RESEARCH PUBLICATIONS
My deep curiosity about the biomedical field led me to explore the immunology of four different medical fields (two infectious diseases and two autoimmune diseases) during my 11 years in various research institutes. This resulted in authorship on 13 publications. The insights I gleaned during this illuminating period continue to serve me well in my work on not only these diseases and the fundamental immunology field but also many other biomedical fields as well. In recent years, I have become more involved in writing papers for busy physicians. This has led to multiple new publications. My effectiveness as a medical writer is evidenced by the markedly increased publication rates of my clients!
Matuszak M, Zidi O, Zevering Y, Perone J-M. Pachymetry as predictive factor of corneal decompensation after phacoemulsification in FECD. Preparation for submission to Cornea.
Retournay L, Goetz C, Zevering Y, Perone J-M. Visual Outcomes of DMEK Relative to Conventional-DSAEK and Ultrathin-DSAEK Defined According to Preoperative and 6-Month Postoperative Central Graft Thickness. Preparation for submission to Cornea.
Perone J-M, Zevering Y, Goetz C. Evolution of intraocular pressure after cataract surgery in nonglaucomatous patients: a posthoc analysis of PERCEPOLIS clinical trial data and narrative review of the literature. Submitted to PLoS One.
Lacroix C, Gedor M, Dinot V, Zevering Y, Perone J-M. Eight-year Outcomes of Descemet Stripping Endothelial Keratoplasty. Preparation for submission to Cornea.
Gan G, Gedor G, Dinot V, Zevering Y, Goetz Y, Perone J-M. Association Between Right Hand Dominance and Right Inter-Eye Asymmetry in Keratoconus Severity. Preparation for submission to Cornea.
Delean S, Goetz C, Zevering Y, Perone J-M. Corneal Central Thickness 6 Postoperative Months After Pseudophakic-DMEK, Triple-DMEK, and Cataract Surgery. Preparation for submission to Cornea.
Lefevre S, Goetz C, Hennequin L, Zevering Y, Dinot V. Effect of body position on suboptimal epinephrine autoinjection. Preparation for submission to Allergy and Astma Proceedings.
Lefevre S, Goetz C, Hennequin L, Zevering Y, Dinot V. Subcutaneous and muscle thicknesses at the anterolateral thigh in adults: predictive patient factors and implications for needle length recommendations. Preparation for submission to Annals of Allergy, Asthma, and Immunology.
Lacroix C, Gedor M, Zevering Y, Perone J-M. Pseudophakic bullous keratopathy cases have worse post-DMEK visual outcomes than Fuchs' endothelial corneal dystrophy cases due to poor graft thinning. Preparation for submission to Acta Ophthalmologica.
Mastrangelo L, Leterrier J, Goetz C, Zevering Y, Perone J-M. Preoperative and Perioperative Factors That Predict Graft Failure 1 Year After Descemet Membrane Endothelial Keratoplasty. Preparation for submission to Cornea.
Perone J-M, Zevering Y, Clavieras C, Takka E, Retournay L, Goetz C. Effect of bilateral cataract surgery and implantation with four intraocular-lens combinations on visual quality of life and uncorrected binocular visual acuity: the ELVIRA-4 multicenter parallel-arm randomized clinical trial protocol. Submitted to BMC Opthalmology 2025.
Leterrier J, Mastangelo L, Goetz C, Zevering Y, Perone J-M. Preoperative and Perioperative Factors That Predict Endothelial Cell Loss 1 Year After Uncomplicated Descemet Membrane Endothelial Keratoplasty. Submitted to Cornea 2025.
Metz D, Gan G, Goetz C, Zevering Y, Moskwa R, Chaussard D, Bloch F, Vermion J-C, Perone J-M. Factors that predict graft detachment after DMEK: a retrospective study of 170 cases. Sci Rep. 2025; 15, 27179. 10.1038/s41598-025-02736-y
Lefevre S, Goetz C, Hennequin L, Zevering Y, Dinot V. Frequencies and predictors of subcutaneous and intraosseous injection with 4 epinephrine autoinjector devices. Ann Allergy Asthma Immunol. 2024;133:194-202. 10.1016/j.anai.2024.05.002
Perone J-M, Goetz C, Zevering Y, Derumigny A. Principal Component Analysis of a Real-World Cohort of Descemet Stripping Automated Endothelial Keratoplasty and Descemet Membrane Endothelial Keratoplasty Cases: Demonstration of a Powerful Data-Mining Technique for Identifying Areas of Research. Cornea. 2024;44;209-220. 10.1097/ICO.0000000000003584
Perone J-M, Luc M-S, Zevering Y, Vermion JC, Gan G, Goetz C. Narrative review after post-hoc trial analysis of factors that predict corneal endothelial cell loss after phacoemulsification: Tips for improving cataract surgery research. PLoS One. 2024;19(3):e0298795. 10.1371/journal.pone.0298795
Falgayrettes N, Patoor E, Cleymand F, Zevering Y, Perone J-M. Biomechanics of keratoconus: two numerical studies. PLoS One. 2023;18:e0278455. 10.1371/journal.pone.0278455
Bichet O, Moskwa, R, Goetz C, Zevering Y, Vermion J-C, Perone J-M. Five-year clinical outcomes of 107 consecutive DMEK surgeries. PLoS One. 2023;18:e0295434. 10.1371/journal.pone.0295434
Perone J-M, Goetz C, Zevering Y. Letter Regarding: Corneal endothelial cell loss after endocapsular and supracapsular phacoemulsification: the PERCEPOLIS randomized clinical trial. Cornea. 2023;42:e13. 10.1097/ICO.0000000000003305
Moskwa R, Bloch F, Vermion J-C, Zevering Y, Chaussard D, Nesseler A, Goetz C, Perone J-M. Postoperative, but Not Preoperative, Central Corneal Thickness Correlates With the Postoperative Visual Outcomes of Descemet Membrane Endothelial Keratoplasty. PLoS One. 2023;18:e0282594. 10.1371/journal.pone.0282594
Gan G, Michel M, Max A, Sujet-Perone N, Zevering Y, Vermin J-C, Zaidi M, Savenkoff B, Perone J-M. Membranoproliferative glomerulonephritis after intravitreal vascular growth factor inhibitor injections: a case report and review of the literature. Br J Clin Pharm. 2022;89:401-409. 10.1111/bcp.15558
Malleron V, Bloch F, Zevering Y, Vermion J-C, Semler-Collery A, Goetz C, Perone J-M. Evolution of Corneal Transplantation Techniques and Their Indications in a Specialized French Ophthalmology Department in 2000–2020. PLoS ONE. 2022;17:e0263686. 10.1371/journal.pone.0263686
Chaussard D, Bloch F, Elnar AA, Zevering Y, Vermion JC, Moskwa R, Perone JM. Identification of the preoperative and perioperative factors that predict postoperative endothelial cell density after Descemet membrane endothelial keratoplasty: A retrospective cohort study. PLoS One. 2022;17:e0264401. 10.1371/journal.pone.0264401
Perone J-M, Goetz C, Zevering Y, Derumigny A, Bloch F, Vermion J-C, Lhuillier L. Graft Thickness at 6 Months Postoperatively Predicts Long-Term Visual Acuity Outcomes of Descemet Stripping Automated Endothelial Keratoplasty for Fuchs Dystrophy and Moderate Phakic Bullous Keratopathy: A Cohort Study. Cornea. 2022. 41:1362-1371. 10.1097/ICO.0000000000002872
Giral JB, Bloch F, Sot M, Zevering Y, Elnar A, Vermion J-C, Goetz C, Lhuillier L, Perone J-M. Efficacy and safety of single-step transepithelial photorefractive keratectomy with the all surface laser ablation SCHWIND platform without mitomycin C for high myopia: a retrospective study of 69 eyes. PLoS One. 2021;16: e0259993. 10.1371/journal.pone.0259993
Bloch F, Dinot V, Goetz C, Zevering Y, Lhuillier L, Perone J-M. Ability of routinely collected clinical factors that predict good visual results after primary Descemet membrane endothelial keratoplasty: a cohort study. BMC Ophthalmol. 2021. 22, 350. 10.1186/s12886-022-02574-w
Perone, J-M, Ghetemme C, Zevering Y, Zaidi M, Ouamara N, Goetz C, Lhuillier L. Corneal Endothelial Cell Loss After Endocapsular and Supracapsular Phacoemulsification. Cornea. 2021;41:714-721. 10.1097/ICO.0000000000002822
Poivret D, Goetz C, Zevering Y, Wilcke C, Noirez V. Effect of patient-led cooperative follow-up by general practitioners and community pharmacists on osteoporosis treatment persistence. Int J Rheum Dis. 2021; 24:912-921. 10.1111/1756-185X.14146
Bellot A, Curien R, Derache A, Delaitre B, Longo R, Zevering Y, Guiilet J, Phulpin B. Oral management in a patient with Gardner-Diamond Syndrome: A case report. Int J Surg Case Reports. 2020. https://doi.org/10.1016/j.ijscr.2020.09.098

Piffer I, Goetz C, Zevering Y, Andre E, Bourouis Z, Blettner N. Ability of Emergency Department Physicians Using a Functional Autonomy-Assessing Version of the Triage Risk Screening Tool to Detect Frail Older Patients Who Require Mobile Geriatric Team Consultation. J Nutr Hlth Aging. 2020; 24:634-641. 10.1007/s12603-020-1378-4
Bischof F, Bins A, Dürr M, Zevering Y, Melms A, Kruisbeek AM. A structurally available encephalitogenic epitope of myelin oligodendrocyte glycoprotein specifically induces a diversified pathogenic autoimmune response. J Immunol. 2004; 173:600-6. 10.4049/jimmunol.173.1.600
Bischof F, Wienhold W, Wirblich C, Malcherek G, Zevering O, Kruisbeek AM, Melms A. Specific treatment of autoimmunity with recombinant invariant chains in which CLIP is replaced by self-epitopes. Proc Natl Acad Sci U S A. 2001; 98:12168-73. 10.1073/pnas.221220998
Zevering Y. Vaccine against Helicobacter pylori? Ann Med. 2001; 33:156-66. 10.3109/07853890109002072. Review.
Zevering Y, Jacob L, Meyer TF. Naturally acquired human immune responses against Helicobacter pylori and implications for vaccine development. Gut. 1999; 45:465-74. 10.1136/gut.45.3.465. [Review]
Zevering Y, Khamboonruang C, Good MF. Human and murine T-cell responses to allelic forms of a malaria circumsporozoite protein epitope support a polyvalent vaccine st
Zevering Y, Khamboonruang C, Good MF. Effect of polymorphism of sporozoite antigens on T-cell activation. Res Immunol. 1994; 145:469-76. 10.1016/s0923-2494(94)80178-9. [Review]
Good MF, Zevering Y. Malaria-specific memory T cells: putative roles of different types of memory responses in immunity and disease. Res Immunol. 1994; 145:455-60. 10.1016/s0923-2494(94)80176-2. [Review]
Zevering Y, Khamboonruang C, Rungruengthanakit K, Tungviboonchai L, Ruengpipattanapan J, Bathurst I, Barr P, Good MF. Life-spans of human T-cell responses to determinants from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium vivax. Proc Natl Acad Sci U S A. 1994; 91:6118-22. 10.1073/pnas.91.13.6118
Zevering Y, Khamboonruang C, Good MF. Natural amino acid polymorphisms of the circumsporozoite protein of Plasmodium falciparum abrogate specific human CD4+ T cell responsiveness. Eur J Immunol. 1994; 24:1418-25. 10.1002/eji.1830240627
Good MF, Zevering Y, Currier J, Bilsborough J. 'Original antigenic sin', T cell memory, and malaria sporozoite immunity: an hypothesis for immune evasion. Parasite Immunol. 1993; 15:187-93. 10.1111/j.1365-3024.1993.tb00599.x
Zevering Y, Amante F, Smillie A, Currier J, Smith G, Houghten RA, Good MF. High frequency of malaria-specific T cells in non-exposed humans. Eur J Immunol. 1992; 22:689-96. 10.1002/eji.1830220311
Good MF, Zevering Y. Peptide analysis of the T cell response to the malaria circumsporozoite (CS) protein. Immunol Lett. 1990; 25:49-52. 10.1016/0165-2478(90)90090-d
Zevering Y, Houghten RA, Frazer IH, Good MF. Major population differences in T cell response to a malaria sporozoite vaccine candidate. Int Immunol. 1990; 2:945-55. 10.1093/intimm/2.10.945
Where my clients have published
Many reputable biomedical subscription and open-access journals


I have edited and written >3000 manuscripts for a broad range of general and specialized journals in the research fields of:
- Biology (immunology, microbiology, molecular biology, biotechnology, zoology)
- Medicine (anesthetics, cardiology, dermatology, diagnostic medicine, ENT, emerging diseases, emergency medicine, endocrinology, gastroenterology, hematology, immunology, intensive care medicine, microbiology, neurology, occupational health, occupational therapy, oncology, ophthalmology, orthopedics, plastic and reconstructive surgery, nephrology, rheumatology, sports medicine, surgery, urology, women's health, veterinary medicine)
- Dentistry (orthodontics, prosthodontics, oral & maxillofacial surgery)
- Epidemiology and public, occupational, and environmental health
Acta Paediatrica
Advances in Medical Sciences
Aesthetic Plastic Surgery
Alimentary Pharmacology & Therapeutics
Allergy
American Journal of Gastroenterology
American Journal of Nephrology
American Journal of Respiratory and Critical Care Medicine
Annals of Allergy, Asthma, and Immunology
Annals of Medicine
Annals of the Rheumatic Diseases
Archives of Toxicology
Arthritis & Rheumatology
Arthroscopy Techniques
BioMedical Research International
BJU International
Blood
BMC Cancer
BMC Musculoskeletal Disorders
BMC Ophthalmology
BMC Pediatrics
BMJ Open
Bone Research
Brain, Behavior, and Immunity
British Journal of Clinical Pharmacology
British Journal of Surgery
British Medical Journal
Burns
Calcified Tissue International
Cancer Immunology Research
Cardiovascular Research
Cell Host & Microbe
Cell Metabolism
Cellular & Molecular Immunology
Circulation
Clinical and Experimental Allergy
Clinical Journal of Sport Medicine
Clinical Microbiology and Infection
Clinical Nutrition
Clinical Orthopaedics and Related Research
Clinical Science
Clinical & Translational Immunology
Clinics in Plastic Surgery
Cornea
Critical Care
Current Medical Research and Opinion
Digestive Endoscopy
Elsevier-published books, book chapters, and university textbooks
EMBO Journal
Emerging Microbes & Infections
Endocrine
European Heart Journal
European Journal of Cancer
European Journal of Immunology
European Urology
European Respiratory Journal
Experimental and Molecular Medicine
Facial Plastic Surgery Clinics of North America
Food Control
Foot and Ankle International
Frontiers in Immunology
Gastroenterology
Gastrointestinal Endoscopy
Gut
Head & Neck
Hepatology
Immune Network
Immunity
Immunology
Immunology Letters
Injury
International Immunology
International Journal of Environmental Research and Public Health
International Journal of Hygiene and Environmental Health
International Journal of Rheumatic Disease
International Journal of Medical Science
International Journal of Surgery Case Reports
International Urogynecology Journal
International Orthopaedics
JACC: Cardiovascular Interventions
JAMA
Japan Journal of Nursing Science
Journal of Affective Disorders
Journal of Allergy and Clinical Immunology
Journal of Bone and Joint Surgery
Journal of Cellular Physiology
Journal of Clinical Immunology
Journal of Clinical Investigation
Journal of Clinical Medicine
Journal of Clinical Microbiology
Journal of Endocrinology
Journal of Ethnopharmacology
Journal of Experimental Medicine
Journal of Gastrointestinal Surgery
Journal of Human Hypertension
Journal of Immunology
Journal of Immunology Research
Journal of Immunotherapy
Journal of Infectious Diseases
Journal of Investigational Allergology and Clinical Immunology
Journal of Investigative Dermatology
Journal of Korean Medical Science
Journal of Laparoendoscopic & Advanced Surgical Techniques and Videoscopy
Journal of Molecular Biology
Journal of Medical Ultrasonics
Journal of Microbiology and Biotechnology
Journal of Neuroinflammation
Journal of Neurointerventional Surgery
Journal of Neurology
Journal of Nutrition, Health, and Aging
Journal of Pediatrics
Journal of Peridontal & Implant Science
Journal of the American College of Cardiologists
Journal of Trace Elements in Medicine and Biology
Journal of Urology
Journal of Virology
Journal for ImmunoTherapy of Cancer
Lancet
Laryngoscope
Lung
Molecules and Cells
Mucosal Immunology
Nature Chemical Biology
Nature Communications
Nature Immunology
Nature Medicine
Nature Neuroscience
Nutrients
Neurology
Osteoporosis International
Pain
Parasite Immunology
Pediatric Blood & Cancer
Pediatric Infectious Disease Journal
Pediatrics
Plastic and Reconstructive Surgery
Plastic and Reconstructive Surgery Global Open
PLOS One
PLOS Pathogens
Proceedings of the National Academy of Sciences of the United States of America
Research in Immunology-Microbes & Infection
Respiratory Research
Retina
Science Translational Medicine
Scientific Reports
Seminars in Immunopathology
Springer e-books
Stroke
Surgical Endoscopy
Ticks and Tick-borne Diseases
Transfusion
Transfusion Medicine and Hemotherapy
Trends in Immunology
Vaccine
Veterinary Microbiology
Veterinary Research
Women's Health Issues
World Journal of Surgery
Testimonials
I have many happy repeat clients, including some I met in my postdoctoral positions more than two decades ago! Here are a few of their testimonials and comments, and also reviewer comments about papers and/or rebuttals I wrote:
"Yinka provides the most professional and reliable editing service I’ve experienced. Her writing is clear and elegant, and she quickly and accurately synthesizes complex information. With her deep knowledge of biomedical sciences, strong critical thinking skills, and attention to detail, she has significantly improved the quality of my manuscripts. I fully trust her work." Department of Life Science, Ewha Womans University, Seoul, South Korea
"Unlike many editing services that focus solely on grammar correction, Yinka provides in-depth support that goes beyond surface-level edits. They thoroughly grasp the logical flow of the manuscript and offer insightful, reviewer-like comments to improve clarity and coherence. Their ability to synthesize complex information and suggest meaningful refinements has been invaluable. I highly appreciate their professionalism, meticulous attention to detail, and deep understanding of immunological research." Department of Biomedical Sciences and Medical Sciences, Seoul national University, Seoul, South Korea
"I appreciate your follow-up and the excellent service." Asan Medical Center, Seoul, South Korea
"this study is well-supported by a comprehensive literature review and has characteristics akin to a review article. I believe this work is valuable because it is easy for readers to understand, and its detailed descriptions offer useful information." Reviewer commenting on a paper I wrote after conducting an extensive literature analysis. The paper has been accepted in Scientific Reports.
"Many thanks for your excellent work. You are au top.👌" Medical Oncology Department, CHR Metz-Thionville, Metz, France
"Thank you for your great work!" Gachon University College of Medicine, Incheon, South Korea.
"This is a well written, very comprehensive study. The topic area is very timely and important as patient-reported outcomes become more and more the standard of care in oncology. The authors have been optimally responsive to the comments and questions of the three reviewers. They have made worthwhile changes to the manuscript that I believe will improve overall understandability and hone the outcome variables somewhat. I am fully supportive of this paper being published in its current format." Reviewer commenting on the revision I conducted and the rebuttal I wrote for a paper I worked on and that was submitted to BMC Oncology.
"With your great helps, I was able to successfully submit the application. Thank you for your help also for offering your help at the last minute too. I really appreciate it!!" Soonchunhyang Institute of Medi-Bio Science, Cheonan-si, South Korea.
"Thank you very much for your excellent revision and for writing the rebuttal letter." Cancer Center, CHR Metz-Thionville, Metz, France
"Thank you so much for your helpful edits and comments!" Department of Anatomy and Cell Biology, Seoul National University College of Medicine, Seoul, Korea
"Thank you so much for your insightful comments. I truly appreciate your detailed feedback, especially regarding the competitor studies and the suggestions for additional experiments. We will carefully review your suggestions to improve the flow of the concepts." Department of Biomedical Sciences, Seoul National University Graduate School, Seoul, Korea
"I am greatly appreciate your meticulous review of our paper and the invaluable feedback you have provided. I am currently incorporating your suggestions and I'm look forward to working together again in the future." Aging Convergence Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea
"Thank you so much for all your comments, and thanks to all your comments, the manuscript got way much better and is now super solid!" Environmental Disease Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea
"The paper was accepted, thank you very much for your wonderful help!" Soonchunhyang Institute of Medi-Bio Science (SIMS) and Soonchunhyang University, Cheonan-si, South Korea
"thank you very much for your excellent revision of the m/s and translation of the added document." Department of Cancer, CHR Metz-Thionville, Metz, France
"Thank you for this new review of our work. All your corrections were very accurate and helpful." Department of Medical Physics, CHR Metz-Thionville, Metz, France
"Thanks to your insightful comments and editing, the paper was published in Cell Reports without significant revision." College of Life Sciences and Biotechnology, Korea University, Seoul, South Korea
"Thanks for all your help with editing and comments. Your perspectives consistently lead us toward new angles that we hadn't thought of before :) I've attached our next paper. Once again, your magic touch with editing would be greatly appreciated. I'm eagerly looking forward to hearing your thoughts on this manuscript. Thank you in advance for your time and expertise." Seoul National University Medical Research Center, Seoul, South Korea
"Thank you for your high quality editing service!" Ewha Womans University, Seoul, South Korea
"I am reaching out to you because we are interested in submitting the attached manuscript as a research article to Medical Physics, and we would greatly appreciate your help with proofreading it. Considering our past collaborations, we believe your insights would be invaluable. We understand that you may have a busy schedule, but if you are available, it would be wonderful to have your support. Although we do not have a specific deadline, we aim to send it as soon as possible, as we believe it addresses a highly relevant and current topic in the field." CHR Metz-Thionville, Metz, France
"Thank you for taking a look at our paper and giving us really helpful feedback. We're glad you caught those important things we missed, and we'll definitely make the changes you suggested. Submitting a paper always hard work, but your help has made it a lot less stressful. Thanks again for your time and expertise." SRC Center for Immune Research on Non-lymphoid Organs, Sungkyunkwan University, Suwon, Korea
"I am deeply impressed by you who work hard with enthusiasm for our research. I am identifying through you new errors that have not been detected despite several internal reviews. Once again, I would like to thank you very much." Ewha Womans University, Seoul, Republic of Korea
"Thank you very much for your great work as always!" Department of Medical Physics, Central Regional Hospital Metz-Thionville, Metz, France
"Thank you for your superb editing!" Nippon Medical School, Tokyo, Japan
"We would like to express very great appreciation to Dr. Yinka Zevering for her valuable and constructive suggestions and for editing this manuscript." Nippon Medical School Musashi-Kosugi Hospital, Kanagawa, Japan
"Bravo à nouveau pour ton travail formidable, Yinka!" [Well done again regarding your excellent work, Yinka!] Ophthalmology Department, Mercy Hospital, Regional Hospital of Metz-Thionville, Metz, France
"Thank you for the attentive and helpful editing!" Ewha Womans University, Seoul, Korea
"Thank you very much for your superb editing as always. Actually, this discussion is not my expert area, so your edit was very helpful." Nippon Medical School, Tokyo, Japan
"Thank you very much for your hard work and for your advice and corrections. And thank you very much for the journal recommendation report, it was very helpful." Department of General and Digestive Surgery, Strasbourg University Hospital, Strasbourg, France
"Many thanks for your insightful editing on our initial manuscript, an editor at Cell reports picked our manuscript" College of Natural Sciences, Chungnam National University, Daejeon, Korea
"Since the last time your work was very helpful, I would like to ask for your help for this article as well." Centre François Baclesse, Esch-sur-Alzette, Luxembourg
"Thanks to your excellent proofreading, the manuscript was quickly accepted by the journal. :)" Seoul National University College of Medicine, Seoul, Korea
"Thank you very much for your careful reviewing of our work. The paper was corrected to consider all your comments, and it was submitted to the Journal of Nuclear Cardiology in January 2022. Thank you VERY MUCH for your precious help in this research project." Department of Medical Physics, Regional Hospital Metz-Thionville, France
"Thank you for the immediate but comprehensive editing. I feel that the manuscript draft has changed completed. Now the edited draft clearly delivers the message I want to say. This is why I contacted you again, and will contact again. THANK YOU!!" College of Life Science and Biotechnology, Yonsei University, Seoul, Korea
"Yinka Zevering a travaillé sur plusieurs de mes articles dans le domaine de l'ophtalmologie et est l'auteur de plusieurs d'entre eux. Elle est une correctrice, rédactrice et traductrice exceptionnelle et est capable de transformer n'importe quel article en un petit bijou. Elle est très exigeante et n'abandonne son travail que lorsqu'il est devenu parfait." [Yinka Zevering has worked on many of my papers in the ophthalmology field and is an author on a number of them. She is an outstanding editor, writer, and translator and can transform any paper into a little jewel. She is very exacting and does not leave her work until it is perfect.] Ophthalmology Department, Mercy Hospital, Regional Hospital of Metz-Thionville, Metz, France
"Dr. Yinka Zevering has edited several papers for our Medical Physics and Radiology department. Her edits are greatly appreciated by our team. Comments she made were always relevant - she does an outstanding work with an in-depth proofreading of each manuscript regardless of the subject matter. Therefore, Yinka has my strong recommendation, she will be of great help for your work." Medical Physics and Radiology Department, Mercy Hospital, Regional Hospital of Metz-Thionville, Metz, France
"Just to inform you that the article was accepted and published. Thank you a lot for the great work and I will surely go with you for futures articles." Departments of Radiotherapy and Physics, Centre François Baclesse, Luxembourg
“I have gotten my papers for medical journals edited by Dr. Yinka Zevering for many years, including basic research, clinical studies, case reports, and review articles. Her editing can be simply stated as “superb” because her editing skills are really reliable for wide areas, and her comments are thoughtful, accurate and faithful. For example, she often kindly suggests a few options with different words and sentences, thus I can choose an option that is closest to my intended meaning. I have never experienced such high quality editing before I knew her. Thanks to her editing, my papers have been accepted to many high impact factor journals. I would like to show my deep appreciation for Dr. Zevering.” Nippon Medical School, Tokyo, Japan
"Brilliant is the first word that comes to my mind to describe the quality of Dr Zevering's medical writing and editing work, regarding ten or so different papers during the last 2 years. Her jobs are always conscientious and relevant, in a wide variety of domains such as nuclear medicine, geriatrics, odontology, cardiology, critical care, ophthalmology... It is a real pleasure to work with her!" CHR de Metz-Thionville, Metz, France
"Yinka has exclusively edited my grant applications as well as my papers over the last 10 years. Her works have been just excellent; the editing itself has been extraordinary, but also she has critically raised many points that could have been concerns for the reviewers. Hence, I strongly believe that her editorial service has significantly contributed to many successes in my grant applications and publications." Harvard Medical School, Boston, USA
"I can confidently recommend you to my colleagues because I am always satisfied with your proofreading :)" Seoul National University College of Medicine, Seoul, Korea
"Your work was really helpful for getting my review paper published in Frontiers of Immunology. I will be happy to work with you again." College of Life Sciences and Biotechnology, Korea University, Seoul, Korea
"Thank you so much for such serious and great edits! All of coauthors also liked your work, which significantly improved the quality of our manuscript." Seoul National University Medical Research Center, Seoul, Korea
"Dr. Zevering is an outstanding editor and experienced scientist whose services have helped me for many years. Although my English is good, I find it very useful to have Yinka take a look at my papers and grants anyway because she is gifted at seeing the big picture and pinpointing how I could improve my writing and argumentation. Her passion for science and her humour also make working with her a pleasure." Centre for Cancer Research, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong
"I met Dr. Yinka Zevering when we worked in the Netherlands Cancer Institute in Amsterdam. She helped me very much in my PhD thesis. Later I asked her if I can hire her when writing my papers and grants. She is an excellent editor and she has helped me a lot in my career." Seoul National University Cancer Research Institute, Seoul, Korea
"Dr. Yinka Zevering is a first-rate editor. She is very careful and accurate and really sees where the paper could be better. I use her analytical review service with every paper now because it gives me so much feedback. Also, because she is a well qualified immunologist, she provides more than language advice; she sometimes gives clever suggestions about experiments or study design that are very helpful. She has a lot of experience and I highly recommend her services.” Aix Marseille Université, Marseille, France
"The paper has been accepted. Thanks for your work! Actually I have introduced you as a good editor and immunologist to my colleagues!" College of Life Sciences and Biotechnology, Korea University, Seoul, Korea
Dr. Zevering, I am happy to give a testimonial. Your service is advanced and serious. It is the best I have ever seen, I will send you my work only. Your editing and comments have helped me publish three papers. I will send you another paper soon. Jinling Hospital, Medical School of Nanjing University, Nanjing, Jiangsu, China
Dr. Zevering edited the English of my paper. Recently, I received my paper after it has been reviewed. The reviewers commented that the standard of English is good. I am really grateful to Dr. Zevering. She also gave me good suggestions on structure. Thanks so much. Medical Research Institute, Tokyo Medical and Dental University, Tokyo, Japan
We have already cooperated with Dr. Zevering two times and her help got us two published SCI papers. She has edited not only the body content but also the response letter, which has helped us a lot. She is careful and accurate, she has checked citations when she has not understood our meaning. We sincerely thank Dr. Zevering for her help. Her service quality is very high, the modification is very good, and the price is fair. I have really recommended her service to all my friends who need it. Osaka University, Osaka, Japan
I recently wrote a review and both reviewers said the language problem is serious. A colleague suggested Dr. Zevering's service. I was very happy with her changes and important comments and questions. Her language was very detailed and in place. The reviewers said they were satisfied with the revision and it was received soon after! Dr. Zevering has provided great help for the publication of the article, thank you! Kyungpook National University Hospital, Daegu, South Korea
"This morning I received the email from the editor about the peer review process; the paper was accepted "as it is". It is the first time that a paper was accepted without revisions and I think your excellent editing process was responsible! Thank you very much." IRCCS Santa Lucia Foundation, Rome, Italy
"I just received the edited paper and would like to say I am very happy with it. Your restructure really improved the transmission of the data. It is now very clear, and understandable for the reader. Thank you for your prompt and excellent work!" Institut Gustave-Roussy, Villejuif, France
"Thank you for your exhaustive and excellent editing. We appreciate your advices and will revise the manuscript according to your recommendations." School of Dentistry, Yonsei University, Seoul, Korea
"Thank you for your perfect editing. We respect your comments and feedback especially." Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China
"Dr. Zevering, my paper was accepted! I am so thankful to you. You are a great editor. You really helped me to see the weakness in my "line of concepts"! :)" Umea University, Umea, Sweden
"I want to tell you our good news - our grant application has been accepted! We are very satisfied with your services and wish to work with you again. We are currently completing a review and would like you to edit it soon." Institut de Biologie Moléculaire et Cellulaire, Université de Strasbourg, Strasbourg, France
"I was very happy with your careful and extensive corrections and very smart comments. I learned a lot about how to structure my manuscript, it will help me a lot when I write my next paper. Thank you." Radboud University Medical Center, Nijmegen, The Netherlands
"The paper was accepted yesterday without changes needed. We couldn't have done it without you!" Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
"You really improved my paper! Your comments are very helpful, especially about the results. I appreciate your professionalism and am very satisfied of your work." Vrije Universiteit Brussel, Brussels, Belgium
"The paper has been accepted. Thank you for your perfect editing." Graduate School of Medicine, Kyoto University, Kyoto, Japan
"I appreciate your rapid editing, preciseness, and the detailed comments on the corrections. I was also very pleased with your feedback and service after the first edit. Your service is excellent. Thank you very much." Heinrich Pette Institute, Hamburg, Germany
"I received the edited manuscript. Your comments about structure helped me a lot. Thank you for your careful work." Juntendo University Graduate School of Medicine, Tokyo, Japan
Privacy & confidentiality
As a scientist who has worked and published in several very competitive fields, I understand how very important it is to safeguard your intellectual property from rivals. Your files are safe with me: all files that are sent to and from the SciMeditor website are fully encrypted by the website platform called Site123, and my computer is protected by Avast Premier and Secureline VPN. In addition, I no longer work in a lab and therefore have no conflicts of interest in relation to any work I receive. I am also the sole editor in SciMeditor: therefore, any work I receive through SciMeditor will only ever be seen by me. I assure you that I will never disclose to any third parties your name, your personal details, your contact information, or the content of any documents you send to me. However, if you would like a special confidentiality agreement, I am happy to accommodate that.
Naturally, if you are happy with my work, please feel free to recommend me to your colleagues or write a testimonial for publication on the Testimonials page of the SciMeditor website. In the case of the latter, I will not indicate any identifying details unless you have approved it in writing.
Naturally, I do collect, via the SciMeditor website and by email, personally identifiable information, namely, your name, e-mail address, and institutional information. This information is collected to facilitate communications with my clients, for quality assurance, and for billing purposes. This information will be stored indefinitely to ensure that future communications with you progress smoothly and rapidly. By entering your personally identifiable information on the Editing Service Order Form, you warrant that I may store and use this information to facilitate further work with you.
Editing Service Order Form
Let's get started!
Please complete the form below or email me at SciMeditor@gmail.com. I will reply by email within 24 hours.
How to pay


On receiving your quote request, I will provide you with a "quote-invoice" by email. If you accept the quote (by email), the quote-invoice will serve directly as an invoice. The quote-invoice will state when the payment is due (usually 1 month after the invoice was sent). The quote-invoice will also contain instructions on how to pay. There are two options:
- Bank transfer: The quote-invoice will provide the required bank details.
- Credit card (Visa, Mastercard, Japan Credit Bureau, American Express, and China Union Pay): credit card payments can be conducted with either PayPal or Stripe. Please choose one and then click on the appropriate logo below. You will be transferred to a secure platform, where you can make your payment.
FAQs
Frequently Asked Questions
- I want to write a paper but I don't have the time. Can you help me?
Yes, I have been a medical writer for over 2 decades, I can help you write and publish your paper! Send me an email to SciMeditor@gmail.com telling me what stage your paper is at and what kind of help you are looking for. I can write a paper from scratch based on the protocol or rudimentary paper and the data. I can also improve or advance your paper with a combination of medical writing and editing.
- How can I order editing services from SciMeditor
It is very easy: review which of the three Editing Packages would best meet your needs and then go to the Order Form, complete your details, upload the files you want me to edit, and click SEND. Please ensure that all of the files you wish to send to me have been fully uploaded before you click SEND. Alternatively, you can contact me by email to SciMeditor@gmail.com. An overview of the ordering process is provided HERE. A detailed description of the ordering process is provided HERE.
- How do I know that you have received my order or email?
Within 24 hours of you submitting the Order Form or an email to SciMeditor@gmail.com, you will receive an email from me addressing your query.
- If I ask for editing, should I send my figures and tables?
Yes, please! They greatly aid the editing work. If you ordered comprehensive editing, I will also edit them if they are in an editable format and check them closely to make sure they are consistent with the manuscript text that relates to them. Please also send the references because I use them when needed in my highly accurate Reference-based approach.
- How will I know when the work is completed?
I will provide you with a deadline beforehand. On or before the deadline, you will receive an email from me with the work and the invoice.
- What about online security and confidentiality?
I fully appreciate how important it is to safeguard your intellectual property. All files that are sent to and from the SciMeditor website are fully encrypted by the website platform Site123, and my computer is protected by Avast Premier and Secureline VPN, so your files are safe with me. I no longer work in a lab and therefore have no conflicts of interest in relation to any work I receive. Moreover, I am the sole editor in SciMeditor: therefore, any work I receive through SciMeditor will only ever be seen by me. Please see the following link for more information. If you would like a special confidentiality agreement, please email me on SciMeditor@gmail.com.
- How and when should I pay?
After I have conducted the required work on your manuscript, I will return it to you with an invoice. You can pay by credit card or bank transfer. The necessary information for paying by all of these methods will be provided in the invoice. If you wish to pay by credit card, you can also do this from the How to pay page, which will send you to the encrypted PayPal or Stripe site. Please pay within 30 days of receiving the invoice.
- Will I receive a payment receipt?
Yes. If you pay by credit card, you will be sent a payment receipt from PayPal or Stripe. If you pay by bank transfer or check, I will send you a payment receipt by email.
- What type of work do you edit?
I edit all types of biomedical documents, including journal manuscripts, grant proposals, books, abstracts, letters, cover letters, responses to referees, and rebuttal letters in the research fields of:
- Biology (immunology, microbiology, molecular biology, biotechnology, zoology)
- Medicine (anesthetics, cardiology, dermatology, diagnostic medicine, ENT, emerging diseases, emergency medicine, endocrinology, gastroenterology, hematology, immunology, intensive care medicine, microbiology, neurology, occupational health, occupational therapy, oncology, ophthalmology, orthopedics, plastic surgery, public health, nephrology, rheumatology, sports medicine, surgery, urology, women's health, veterinary medicine)
- Dentistry (orthodontics, prosthodontics, oral & maxillofacial surgery)
- Epidemiology
- What is the Analytical Review Service?
The Analytical Review Service provides an in-depth and detailed critical analysis of your paper. It serves as a before-peer review that picks up the weaknesses in the study report, so that you can address them before the peer reviewers see them. It is written after I have comprehensively edited the paper. It comes in the form of a report where I first check that a long list of criteria are met, including clear explanation of the study rationale, study design, and study limitations. The second part of the report lists the problems that could not be resolved during editing, such as lack of clarity about the key message of the paper, study objectives, and how the study contributes to the field. Key recommendations are provided. See this link for more information.
- Do you adhere to biomedical reporting guidelines?
Yes. I edit according to the appropriate guideline and will inform you if any reporting elements are still missing after I have worked on your paper.
- How do I see the changes in my edited manuscript?
You will receive a file containing the manuscript with the changes visible under Track Changes. Track Changes is a feature of Microsoft Word and can be found by clicking the Review menu. When Track Changes is activated, all changes in the manuscript are visible, including insertions. An example is given here. You can accept or reject changes by right clicking on the highlighted text and choosing the appropriate option from the pop-up menu.
- If I make revisions, will I have to pay to get my manuscript edited again?
- If you chose the Basic Editing package for the first edit, you can get a revision edit for 50% of the Basic Edit rate.
- If you chose the Comprehensive Editing package for the first edit, you are eligible for one free re-edit of previously edited work and 10% of new added text.
- If you chose the Comprehensive PLUS Ongoing Support Until Publication package, you will receive unlimited revisions until your paper has been approved for publication.
An exception to these rules is if the manuscript has been so substantially altered that it bears little resemblance to the original manuscript. In this case, I may deem the work to be a new manuscript and will have to charge usual or discounted rates. Please see the SciMeditor policies page for more information.
- Do you ensure that the manuscript meets the word count limits that are required by the journal or granting body?
All papers written by me will meet all journal requirements. With regard to editing work:
- If you select the Basic Editing package, word limits will not be checked during editing.
- If you select the Comprehensive package, I will ensure that the Abstract meets the word count limit. If that requires substantial reduction, I will provide two versions of the fully edited Abstract, one before and one after word count reduction. I will also try to ensure that the manuscript body also meets the word count limit. However, if the word count markedly exceeds the word count limit, I will inform you that the manuscript body exceeds the word count limit and advise you to revise the manuscript to reduce the word count.
- If you select the Comprehensive PLUS Ongoing Support Until Submission package, I will ensure that all word count limits are met in the first edit. In cases of substantial reduction, I will explore with you which elements could be deleted or reduced.
- Can you supply an editing certificate that I can send to the journal?
If I have checked the paper in its entirety and no further changes have been made, I can provide a PDF editing certificate. I do not provide editing certificates for the Basic editing service because this service only provides basic language checks.
- Do you check for plagiarism?
I do not routinely check for plagiarism but my Reference-based approach means that I sometimes find sentences that are inadvertently similar to sentences in the cited publications. In this case, I paraphrase the sentences so that the key message is retained but the wording is different. I have never encountered a case of substantial plagiarism but if I did, I would return the paper to the authors asking them to rewrite the section in question. If you have any concerns, you can check your work with a plagiarism detection program, such as those provided by many institutions. Alternatively, you can check by placing individual sentences into Google.
- Can you advise me about which journal I should send my paper to?
I recommend to use journal finder programs. There are several free programs online. They include the Journal Finder from Elsevier, the Journal Suggester from SpringerNature, the Journal Finder from Wiley, the Journal Suggester from Taylor & Francis, and Journal Author Name Estimator (JANE), JournalGuide and Researcher.Life, which give journal recommendations across publishers. If you have a subscription to the EndNote reference manager, you can also access their Manuscript Matcher. However, if you would like me to provide a journal recommendation report, please indicate this in the Order Form and I will provide you with a quote.
Basic Editing package
This package is suitable for well-written, fully comprehensible, and unambiguous papers that are ready for submission/re-submission pending light proof-reading. It corrects:



Re-edits of Basic Editing manuscripts are charged at a 50% DISCOUNT.
Click HERE to see an example of the Basic Editing service.
Prices for the Basic Editing package* | |
|---|---|
Price per word Regular (7-day) | Price per word Express (72 hours) |
US$0.06 / €0.05 | US$0.07 / €0.06 |
*Euro prices may change depending on the exchange rate between US dollars and the Euro
To estimate how it will cost to edit your manuscript with the Basic Editing service, simply multiply the total number of words in your manuscript (without references) by the price per word shown above. For example, if your manuscript is 2000 words (without references), Basic Editing at the Regular rate will cost US$120 (2000xUS$0.06) or US$140 at the Express rate (2000xUS$0.07).
Comprehensive Editing package
This package is suitable for any academic text written by researchers who are non-native speakers of English. It provides:

- the key messages, impact, and significance of the work are immediately obvious to the reader
- the aim and rationale of the study or work are clear and are expressed consistently throughout the text
- the concepts flow smoothly and logically from paragraph to paragraph and from section to section
- there is no ambiguity or inclusion of less relevant concepts or data that could cause reader confusion
- the methods are described with the appropriate amount of detail needed for reproducibility
- there are no missing data and no discrepancies in the way the data are described in various sections of the work
- the statistics that are used are appropriate and are described correctly
- the figures, figure legends, and tables are spelled and formatted correctly and consistently







Click HERE to see an example of the Comprehensive Editing service.
Prices for the Comprehensive Editing package* | |
|---|---|
Price per word Regular (7-day) | Price per word Express (72 hour) |
US$0.10 / €0.09 | US$0.14 / €0.125 |
*Euro prices may change depending on the exchange rate between US dollars and the Euro
To estimate how it will cost to edit your manuscript with the Comprehensive Editing service, simply multiply the total number of words in your manuscript (without references) by the price per word shown above. For example, if your manuscript is 2000 words (without references), Comprehensive Editing at the Regular rate will cost US$200 (2000xUS$0.10) or US$280 at the Express rate (2000xUS$0.14).
* Note that the Analytical Review Service provides an in-depth detailed analysis of the strengths and weakness of the manuscript.
** Word counts will be met unless the word count markedly exceeds the word count limit. At that point, further word count reduction becomes risky: I may inadvertently delete important information. Large word count reduction is also very time-consuming. In this case, I will inform you that the manuscript body exceeds the word count limit and that I advise you to revise the manuscript to reduce the word count.
Comprehensive PLUS Ongoing Support Until Publication package
This package is ideal for authors who desire ongoing support until submission. It offers ALL of the advantages of the Comprehensive Editing package AND



Prices for the Comprehensive PLUS Ongoing Support Until Submission package* | |
|---|---|
Price per word Regular (7-day) | Price per word Express (72 hour) |
US$0.17 / €0.15 | US$0.19 / €0.17 |
*Euro prices may change depending on the exchange rate between US dollars and the Euro
To estimate how it will cost to edit your manuscript with the Comprehensive PLUS Editing service, simply multiply the total number of words in your manuscript (without references) by the price per word shown above. For example, if your manuscript is 2000 words (without references), Comprehensive PLUS Editing at the Regular rate will cost US$340 (2000xUS$0.17) or US$380 at the Express rate (2000xUS$0.19).
Reference-based Writing and Editing
An essential approach to sensitive and high-fidelity writing and editing

(Note: I do not routinely check or edit references)
When I am writing a paper or editing one, I often Google individual references and sometimes other papers to make sure that my writing/editing is accurate. Specifically, I will check the Abstract (or, if readily available, the online paper) of the cited paper or related papers. This ensures that:
(i) the manuscript faithfully conveys the author's intended meaning, and
(ii) the concepts in the paper reflect the up-to-date literature.
An example of how this approach works is as follows. Here, the author has stated in the Introduction:
"In Germany, 14,000 patients are diagnosed with multiple sclerosis every year and require TNFalpha treatment [ref1]."
This could be interpreted as:
"Every year, 14,000 patients with multiple sclerosis will require treatment with TNFalpha [ref1]."
OR
"In Germany, 14,000 patients are newly diagnosed with multiple sclerosis every year, many of whom will eventually require TNFalpha treatment [ref1]" (this is the correct statement)
Such ambiguity impedes the smooth flow of concepts and can obscure the key messages of the manuscript.
This reference-based approach also allows me to occasionally pick up referencing mistakes (such as citing the wrong paper) and to rewrite texts that resemble passages in the published paper too closely. The latter is a common inadvertent error made by authors who have difficulties writing in English but it can lead to rejection by the journal on the basis of plagiarism software, which many journals use routinely.
Reference-based Editing means that I can also offer versions of the original text that explain the point more precisely. For example, "Effort to improve antibiotic use has started a few years ago in Sri Lanka[ref9]". To improve this, I will check reference 9 and suggest to the author that the following reformulated sentence may be more suitable: "To improve antibiotic use in Sri Lanka, the government launched their first antibiotic stewardship program in 2011 [ref]."
Because I often rely heavily on references in my work, I strongly encourage authors to supply the relevant bibliography.
Occasionally, even with my reference-based approach, it is not possible to discern what the author means. In such cases, I will explain why the sentence is confusing and, if possible, rewrite the text according to an educated guess: this rewritten text will be marked by an editor comment. I can sometimes also provide one or more alternatives in the comments: this allows the author to choose which version best captures their intended meaning.
Biomedical study reporting guidelines
Essential guides to writing high-quality scientific reports
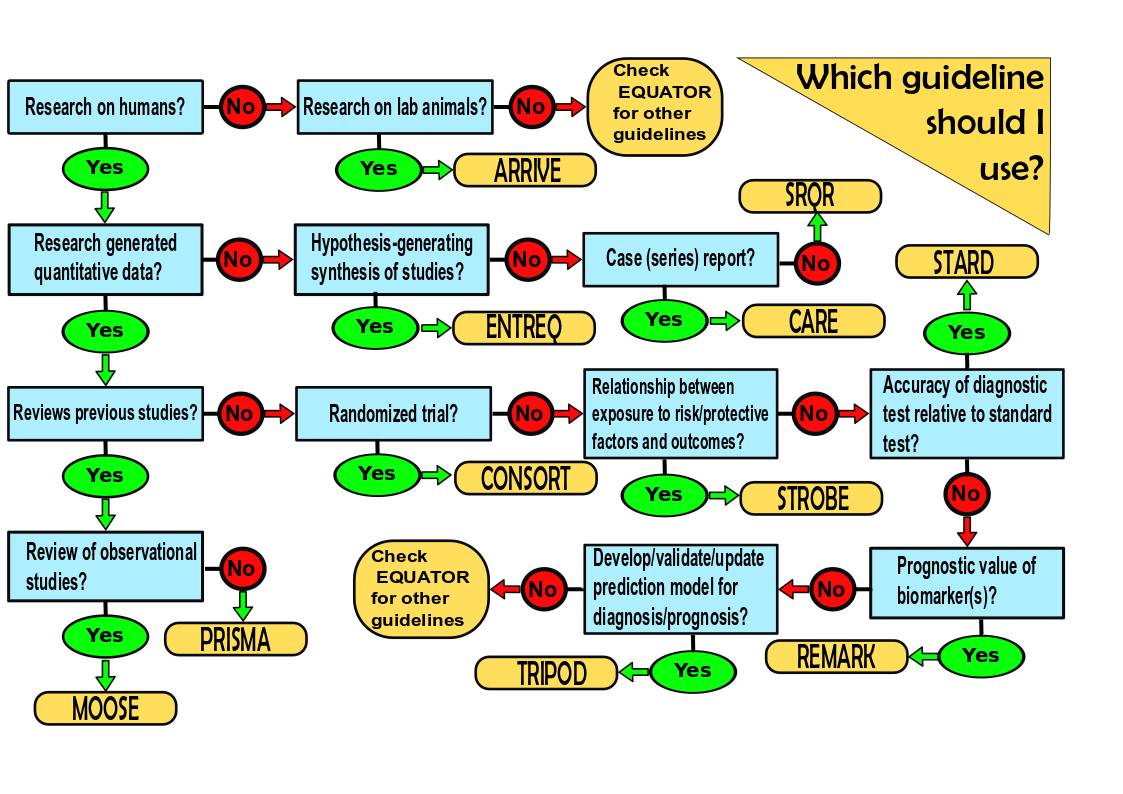 Reports of biomedical studies such as randomized clinical trials, observational research studies, case series studies, in vivo animal studies, systematic reviews, and meta-analyses often lack detail regarding key elements of the study, such as its design, how patients were selected, and how key outcome variables are defined. This makes it difficult for time-poor reviewers and readers to quickly glean the essentials of the paper, assess the quality of the study, and compare it to similar studies.
Reports of biomedical studies such as randomized clinical trials, observational research studies, case series studies, in vivo animal studies, systematic reviews, and meta-analyses often lack detail regarding key elements of the study, such as its design, how patients were selected, and how key outcome variables are defined. This makes it difficult for time-poor reviewers and readers to quickly glean the essentials of the paper, assess the quality of the study, and compare it to similar studies.
Consequently, high-quality studies may be overlooked or misunderstood and may end up being published in less prestigious journals.
To address these problems, biomedical study reporting guidelines have been generated. They indicate the key reporting elements and the order in which these elements should be reported. Most reputable journals require adherence to these guidelines. They are listed in The Equator (Enhancing the QUAlity and Transparency Of health Research) Network and include:
- CONSORT for reporting randomized clinical trials
- PRISMA for reporting reviews of randomized clinical trials
- STROBE for reporting observational studies such as cohort, cross-sectional, and case-control studies*
- MOOSE for reporting reviews of observational studies in epidemiology
- ARRIVE for reporting in vivo animal studies
- SRQR for reporting qualitative research
- ENTREQ for reporting syntheses of qualitative research
- REMARK for reporting studies on the ability of tumor markers to predict prognosis
- STARD for reporting diagnostic accuracy studies
- CARE for reporting case reports
I have extensive experience with these guidelines and adhere to them as much as possible during editing. I occasionally reorganize the Methods and Results sections so that they meet guideline requirements. I also indicate with editor comments where information required by the guideline is missing.
* I have constructed a simple version of STROBE that includes key elements in CONSORT: please click here.
Detailed ordering information
Details of ordering a SciMeditor Editing package with or without an Analytical Review
First, go to the Editing Packages page and determine which of the three packages best meet your needs. If you want a detailed critical analysis of your manuscript, take a look at the Analytical Review Service.
Then go to the Order Form and choose the package and turnaround time that suits your needs. Upload all of the documents that you want to have edited. These files may include, for example, your study report, the cover letter, and the tables. Please also upload the figures and references because they greatly aid the editing work. The references will not be edited. The figures will be checked for errors and discrepancies.
You can also simply attach your documents to an email to SciMeditor@gmail.com that tells me which package and turnaround time you prefer.
Within 24 hours, I will review your order and reply to your email. If I have no questions, I will ask you to send me an email to SciMeditor@gmail.com confirming that I can start editing your work.
I will then edit your paper. Depending on the package chosen, I will edit for English language only (the Basic package) or comprehensively edit the manuscript so that the concepts flow clearly and smoothly, there are no discrepancies between sections, and the manuscript meets journal and/or other guidelines (the Comprehensive and Comprehensive PLUS packages). In the latter two packages, the ultimate aim is to produce a manuscript that is so clear that a lay scientist will rapidly and fully understand the significance of your work. The two packages differ in that the PLUS package also includes unlimited free edits, reducing the word count to meet journal requirements, and free cover letter editing.
I use "Track Changes" in Word and add comments to explain why I made certain changes or to indicate where additional information is needed. Here is an example of comprehensive editing:

If you ordered an Analytical Review, I will complete the Analytical Review form after thoroughly editing the manuscript according to the selected Editing Package specifications.
I will then send you the edited work together with an invoice that explains how you can pay. You have the choice of paying by credit card, bank transfer, or check. You can also pay by going to the How to pay page in the SciMeditor website.
You should then accept the changes that you like in the edited manuscript and make any necessary clarifications.
If you chose the Comprehensive or Comprehensive PLUS package, you can send the revised manuscript back to me via email (SciMeditor@gmail.com). The re-edits must be returned within 1 year of receipt of the first edited manuscript.
If you have any questions or problems at any point, please feel free to contact me on SciMeditor@gmail.com. I will do my best to help you.
Example of Basic Editing
The original text, which is relatively well written, looks like this: After Basic Editing, the text looks like this:
After Basic Editing, the text looks like this:

After accepting all changes, the text looks like this:

Example of Comprehensive Editing
The original text looks like this:

The text looks like this after Comprehensive editing:

Overview of malaria vaccines

Updated November 2018
Overview of immunopathology in rheumatoid arthritis
 Updated November 2018
Updated November 2018
Overview of immunopathology in multiple sclerosis
 Updated November 2018
Updated November 2018
Overview of pathology caused by Helicobacter pylori
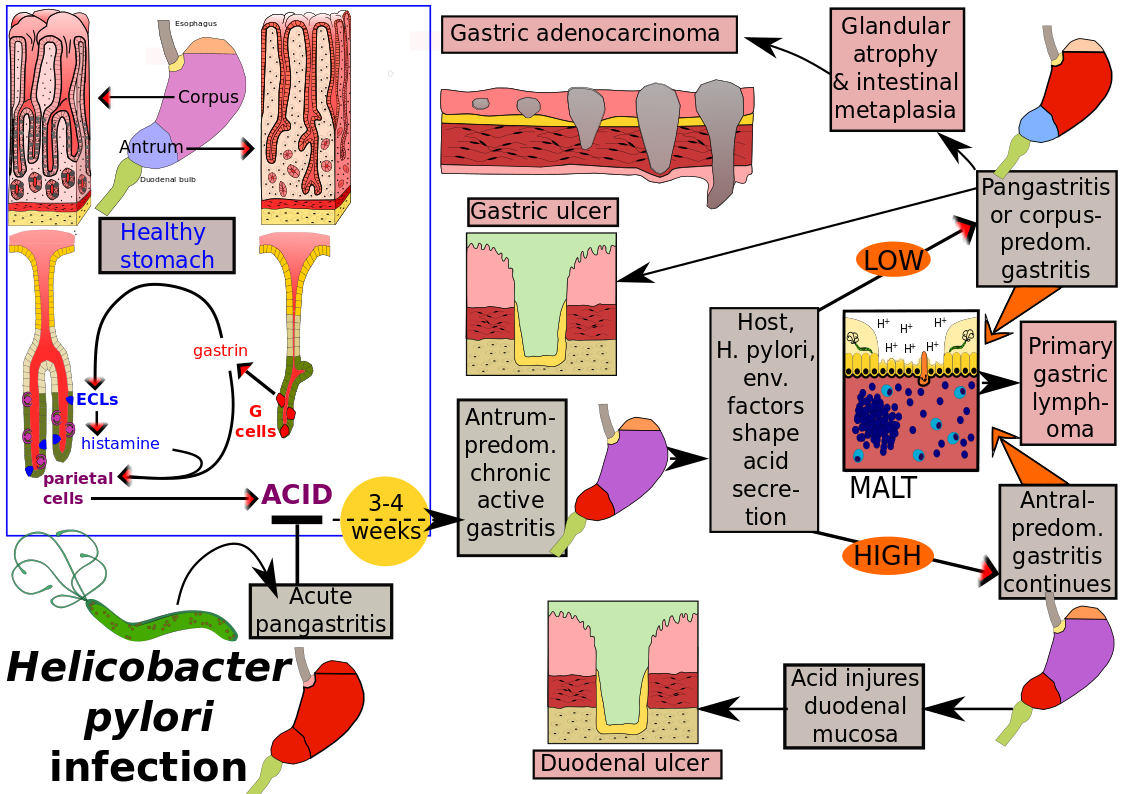 Updated November 2018
Updated November 2018
Four common fatal mistakes when writing a paper: No. 2
My many years of editing experience have led me to identify four very common and unfortunately fatal mistakes that scientists and physicians make when they write their paper. These mistakes are so serious that you risk immediate rejection if you make even just one of them. Here I will describe one of these mistakes and show how you can avoid it.
FATAL MISTAKE 2: Unclear what the study adds to the body of knowledge 
Example: study in human cadavers that examined the ability of a new rod and screw system to fuse lumbar vertebrae (L1-L5).
Introduction: describes the reasons for lumbar fusion. Does not mention other lumbar fusion methods.
Results: new system stably fuses L2 to L3, not so good with other pairs or more extensive fusions.
Discussion: discusses at length how to improve the system to make it more useful. No mention of how new system could compare to other existing systems.
HOW NOVEL IS THE NEW SYSTEM? WHAT DOES THE STUDY ADD TO THE FIELD?
Why is this a serious error?
Because papers should be written for a general audience and even specialists in the field will want some discussion about existing modalities.
How can you avoid this mistake?
After writing your paper, ask yourself: have I made clear how my study adds to the field in:
Abstract 
Introduction 
Discussion 
Collect the texts on background on one page. Do these texts clearly present how the paper adds to the field?
Four common fatal mistakes when writing a paper: No. 3
My many years of editing experience have led me to identify four very common and unfortunately fatal mistakes that scientists and physicians make when they write their paper. These mistakes are so serious that you risk immediate rejection if you make even just one of them. Here I will describe one of these mistakes and show how you can avoid it.
FATAL MISTAKE 3: Not openly disclosing that your study is confirming/testing the findings of another study
Example: murine study on a new angiotension-converting enzyme (ACE) inhibitor in chronic kidney disease.
Introduction: “Several studies have shown that ACE inhibitors in CKD are effective [refs 5,6,8]”

Discussion: “A study similar to ours showed that [the new ACEi] is also effective for CKD [ref 5].”
Why is this a serious error?
Because it is annoying for a reviewer to find near the end of the paper that the study is not novel. In addition, confirmatory studies are an important part of science and are generally respected as such, so hiding the confirmatory nature of your study is unnecessary.
How can you avoid this mistake?
After writing your paper, ask yourself: have I made it absolutely clear that my study aimed to test the reliability of the results of another similar study in:
Abstract 
Introduction 
Discussion 
Conclusion 
Four common fatal mistakes when writing a paper: No. 4
My many years of editing experience have led me to identify four very common and unfortunately fatal mistakes that scientists and physicians make when they write their paper. These mistakes are so serious that you risk immediate rejection if you make even just one of them. Here I will describe one of these mistakes and show how you can avoid it.
FATAL MISTAKE 4: Not clearly describing patient selection and comparator groups 
Example: monocentric retrospective study comparing a nutrition program to standard care after radical surgery for advanced gastric cancer.
Methods: “This cohort study included patients with advanced non-metastatic gastric cancer who underwent radical surgery in 2014-2015.”
Results: “Patient characteristics are shown in Table 1. The nutrition program improved variables 1, 2, and 3 but not variables 4, 5, and 6.”
This paucity of information leaves the reader with many questions:
• Were the patients ALL CONSECUTIVE patients (or randomly selected, or a convenience series?)
• What were ALL the inclusion and exclusion criteria?
• How many patients were excluded, and for which reason? How did the nutrition-program and standard-care patients compare in terms of numbers excluded for certain criteria?
• How did the nutrition-program patients compare to the standard-care patients in terms of baseline and perioperative variables?
Why is this a serious error?
Because it is essential to disclose the information that will show if there is patient selection bias that could affect the interpretation and generalizability of your results.
How can you avoid this mistake?
By consulting the relevant biomedical study reporting guideline. In this example, STROBE would be appropriate. A randomized controlled trial would require CONSORT. The relevant guideline for your study can be found here. I have also constructed a simplified STROBE guideline that contains relevant CONSORT elements.
Example of track changes

The text looks like this after Comprehensive editing:

Simple version of STROBE with CONSORT elements
In my experience, STROBE does not list all of the elements needed for accurately and fully reporting observational studies. To address this, I added some CONSORT elements to the existing STROBE checklist for my own purposes.



Twenty common serious mistakes when writing a paper
These mistakes are very commonly made when authors write their scientific paper. They increase the risk of rejection, especially when many of these errors are present. The following table will list these mistakes, explain why they are problematic, and how they can be avoided.
In addition to the hints given here, I should point out that many of these mistakes can be avoided by checking the relevant study reporting guideline for the reporting structure and elements that your paper will need. I have also created a simplified guideline for reporting human observational studies that includes relevant STROBE and CONSORT guidelines.
Part of paper | The mistake | Comment | How to avoid this mistake |
| ABSTRACT | 1. Abstract lacks one or more key elements
| The Abstract is the most read part of your paper. It is essential that it contains ALL of the key elements and messages of your paper. | Check that ALL elements required are included. CONSORT for Abstracts gives good guidelines for not just randomized controlled trials but also observational human studies in general |
| INTRODUCTION, DISCUSSION, REVIEWS | 2. Studies cited in these literature-based papers/paper parts are not fully described (a) Type of study is unclear (e.g. RCT, cross-sectional, case report, in vitro, animal study, in silico study) | This makes it difficult for the reader to determine the quality of the evidence for the claim being made. Is it reliable (RCT, large prospective cohort study) or weak (case report, in vitro study?) | Check that the study type and its important elements are fully described |
(b) Type of subject unclear:
| This makes it difficult to see how comparable similar studies are | Check that the study type and its important elements are fully described | |
| 3. References are incorrect | This mistake is unnecessary and it gives the impression of sloppiness. That could make the reviewer distrust everything about the paper. | Check that all references are appropriate and correctly cited | |
| 4. References are not the ORIGINAL source of the data supporting the claim/statement in your paper | This can lead to unreflected dogmas in the field that have not actually been tested experimentally. These unchallenged dogmas can be quite destructive to the progression of science | Try as much as possible to make sure that the cited paper provides original data supporting the statement | |
| 5. Accidental plagiarism | This is because of copy and paste and then not reformulating. Copy-paste is a common technique for extracting information from the literature but if not rewritten, it is often clearly detectable to readers and may trigger journal plagiarism software | Put the copied-pasted text in a color and then, when revising the text, make sure that only one in three/four words remains that color | |
| METHODS | 6. No/poor ethics section | Having a well-written and detailed ethics section gives the reader confidence that you understand the importance of ethics in biomedical science | All studies: "This study was approved by [the appropriate] ethics committee/institutional review board" Human studies: "This study adhered to the tenets of the Declaration of Helsinki and its revision"; "This study adhered to Good Clinical Practice guidelines"; "All patients provided written/oral informed consent to have their data included/ to participate in the study" Animal studies: "This study was conducted according to international [e.g. Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International)], national, and/or institutional guidelines for humane animal treatment and complies with [relevant] legislation" |
| 7. Human study design not stated or incorrect (e.g. Cohort when it is cross-sectional) | Disclosing the study type helps the reader to quickly estimate the likely quality of your study findings. Not adding this information or using the wrong label may make the reviewer think that your understanding of study design could be limited | If unsure, consult the Equator Network to determine what type of study your study is | |
| 8. Not stated when the study in humans was conducted (e.g. January 2014-March 2015) | Was the study performed last year or two decades ago? This is particularly important for clinical studies because clinical practice can change very markedly over time | Make sure that you indicate when the study was performed in the Abstract and Methods | |
| 9. Experimental timelines unclear | e.g. when after the intervention did treatment start and when were samples taken? How long was follow-up? These experimental details should be described very clearly so that the reader knows how the experiment/study proceeded | Clearly describe these experimental details in the Abstract and Methods. Consider using a schematic depiction of the experimental timeline if multiple intervention timepoints and/or sampling timepoints are used | |
| 10. Detailing patient numbers in the Methods section e.g. “1022 patients were enrolled and 19 were excluded” | The Method section in human studies should only describe HOW the patients were selected | Only describe patient numbers in the Results section. Animal/well/cell numbers can be indicated in the Methods if the same number are used for all experiments. If varying animal/well/cell numbers are used, indicate this in the figure legends | |
| 11. Primary and secondary outcomes are unclear | The primary outcome MUST be clearly indicated because the power calculation determining the optimal study sample size should be based on being able to detect a a significant different regarding the primary outcome. This is often a confusing area in the Methods | Make sure to clearly distinguish between primary and secondary outcome measures in the Abstract and Methods | |
| 12. No power calculation | Does the study have enough power to detect a difference in primary outcome? Otherwise, the study is essentially useless | Consult a statistician to determine the optimal sample size. If necessary, a post-hoc analysis can be performed (but a priori power size calculations are better) | |
| 13. Statistics section incomplete/unused stats methods mentioned | This makes the reviewer think you do not understand your statistics | Make sure the Statistics section clearly explains ALL statistics you used and which analyses were performed | |
| METHODS & RESULTS | 14. Use of uninformative experimental group names | e.g. Group 1, 2, and 3. The reader has to work hard to remember which is the control, which is intervention A, which is intervention B etc | Generally, group names are NOT needed. If absolutely necessary, use short, memorable, and completely distinguishable groups names |
| 15. Use of complicated experimental group names | e.g. a study in rats where the sciatic nerve is denervated surgically and then half of the rats start exercise training (ET) at 2 or 6 weeks. Groups are called Den2w, Den6w, Den2wET, Den6wET. It can be very confusing for the reader | Generally, group names are NOT needed. If absolutely necessary, use short, memorable, and completely distinguishable groups names | |
| RESULTS | 16. Describing experiments that have not been mentioned in the Methods (or vice versa) | Many readers will quickly skim through the Methods before moving onto the Results. If an experimental method is suddenly mentioned in the Results but the method was not detailed in the Methods, this can confuse the reader | Make sure that all methods used to get the results are actually described in the Methods (and vice versa) |
| 17. Describing data that do not relate directly to the study objective. Often people do this because these data are not sufficient to write a whole paper about | e.g. cohort study examining whether 800 patients with rheumatoid arthritis on MTX mount good antibody responses to flu vaccine A. A subgroup of 20 patients is examined for T-cell responses to flu vaccine B antigen. The subgroup analysis will distract the reader from your main findings, disrupt the smooth flow of concepts, and cause confusion | Make sure that ALL data relate directly to the study question | |
| 18. Unclear how many patients/animals/wells per experiment, and how many times the experiment was performed | This is Science101 basic information that should always be included because it shows how reliable the findings are | Always indicate how many patients/animals/cells/wells were used for each experiment in the Results (human studies) or figure legends (other studies) | |
| 19. Inconsistencies between the Results section (and/or Abstract) and the data shown in the tables and figures | e.g. Results: “Of the 93 patients who received the study drug, seven (7.5%) had developed new-onset hypertension at 3 years.” Table 1:
| Always check that all data cited in the Abstract and Results match completely with the data in the figures and tables | |
| DISCUSSION | 20. No Study Limitations section | No study is perfect - all have flaws e.g.
| It is essential that you are completely open about the limitations of your study. It makes you look trustworthy and shows that you are really interested in answering the scientific question |
























