Biomedical study reporting guidelines
Essential guides to writing high-quality scientific reports
 Reports of biomedical studies such as randomized clinical trials, observational research studies, case series studies, in vivo animal studies, systematic reviews, and meta-analyses often lack detail regarding key elements of the study, such as its design, how patients were selected, and how key outcome variables are defined. This makes it difficult for time-poor reviewers and readers to quickly glean the essentials of the paper, assess the quality of the study, and compare it to similar studies.
Reports of biomedical studies such as randomized clinical trials, observational research studies, case series studies, in vivo animal studies, systematic reviews, and meta-analyses often lack detail regarding key elements of the study, such as its design, how patients were selected, and how key outcome variables are defined. This makes it difficult for time-poor reviewers and readers to quickly glean the essentials of the paper, assess the quality of the study, and compare it to similar studies.
Consequently, high-quality studies may be overlooked or misunderstood and may end up being published in less prestigious journals.
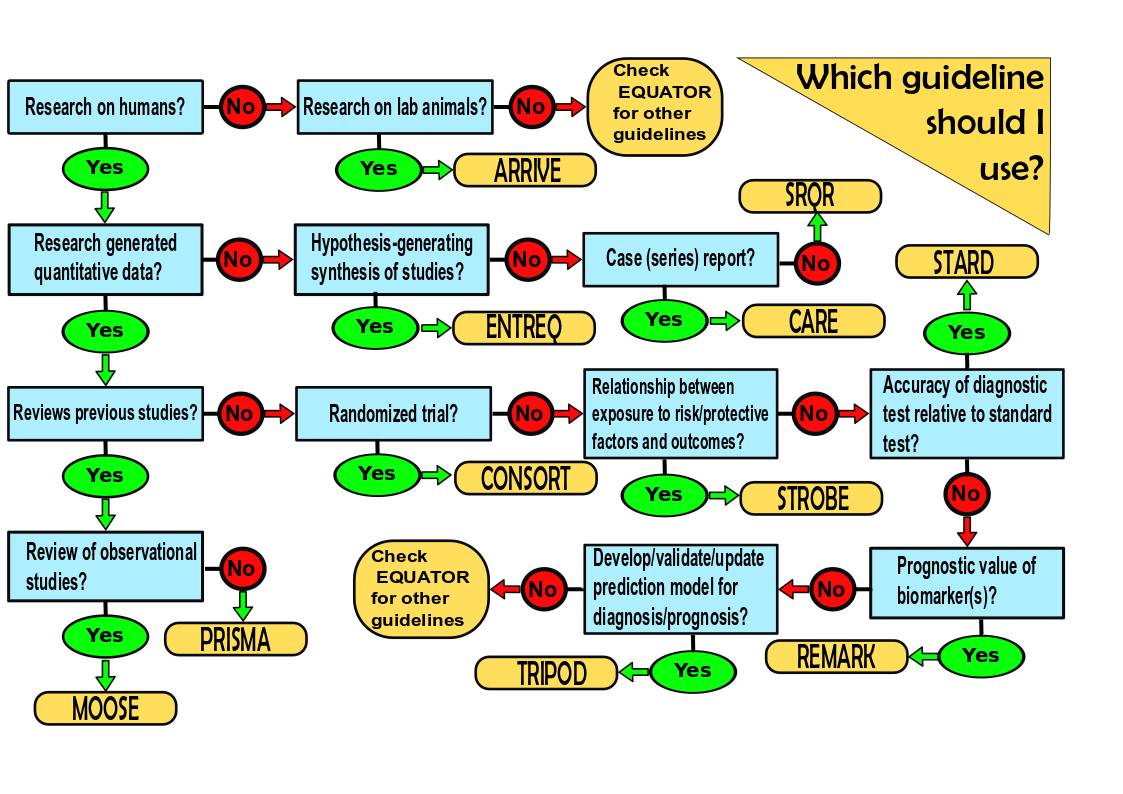
To address these problems, biomedical study reporting guidelines have been generated. They indicate the key reporting elements and the order in which these elements should be reported. Most reputable journals require adherence to these guidelines. They are listed in The Equator (Enhancing the QUAlity and Transparency Of health Research) Network and include:
- CONSORT for reporting randomized clinical trials
- PRISMA for reporting reviews of randomized clinical trials
- STROBE for reporting observational studies such as cohort, cross-sectional, and case-control studies*
- MOOSE for reporting reviews of observational studies in epidemiology
- ARRIVE for reporting in vivo animal studies
- SRQR for reporting qualitative research
- ENTREQ for reporting syntheses of qualitative research
- REMARK for reporting studies on the ability of tumor markers to predict prognosis
- STARD for reporting diagnostic accuracy studies
- CARE for reporting case reports
I have extensive experience with these guidelines and adhere to them as much as possible during editing. I occasionally reorganize the Methods and Results sections so that they meet guideline requirements. I also indicate with editor comments where information required by the guideline is missing.
* I have constructed a simple version of STROBE that includes key elements in CONSORT: please click here.

